Accelerate your CRISPR breakthrough with an integrated CDMO workflow
Companies developing gene editing therapies often face challenges or delays due to the complexities of GMP manufacturing and the need to coordinate multiple vendors.
Streamline your path with BIOVECTRA and Agilent
BIOVECTRA, now part of Agilent, offers a single source for gene-editing technologies. This simplifies the complexity of multi-vendor coordination with an integrated path from process development to GMP manufacturing, fill/finish, and analytics.
Addressing challenges of gene editing manufacturing
Coordinating manufacturing for synthetic sgRNA, plasmid DNA, mRNA, and other components across multiple CDMOs often leads to delays, regulatory hurdles, and quality risks. Each handoff—between partners with their own facilities, timelines, and standards—adds complexity, especially during shipping, and can slow progress to clinic.
Agilent and BIOVECTRA eliminate these barriers with a fully integrated model. Together, we streamline operations, reduce risk, and accelerate your path to market.

Overcoming the challenges of gene-editing manufacturing. Manufacturing a gene-editing drug product involves multiple complex steps. Outsourcing each step to a different vendor can cause significant challenges and often lead to delays. 16+ months from start to finish is not uncommon.
Partnering with an integrated CDMO to handle the entire process helps minimize or even eliminate many of these issues. Every stage – from manufacturing to final QA release – is orchestrated by our team for the fastest path to market, targeting 12 months or less.
Two Partners. One Integrated Path.
Agilent and BIOVECTRA work in close coordination to deliver a fully integrated gene-editing solution—combining capabilities into one streamlined process. The Agilent Nucleic Acid Solutions Division (NASD), with two GMP facilities in Colorado, efficiently onboards and scales multiple programs, performing process development, synthesis, and purification of CRISPR sgRNA using the ClinGuide platform. This includes process development to optimize purity and yield, supported by a proprietary orthogonal chromatography method that achieves over 80% purity and aligns with regulatory expectations.
BIOVECTRA complements this workflow with downstream services, including LNP formulation, fill/finish, and comprehensive QC and analytics. As the gene-editing field evolves, BIOVECTRA is equipped to support both ex vivo and in vivo workflows. With the addition of Agilent’s GMP sgRNA manufacturing, BIOVECTRA extends its capabilities across a broad range of therapeutic applications. Its facilities are purpose-built for flexibility and scale, leveraging proven mRNA and LNP processes to meet the demands of next-generation CRISPR programs. Phase-appropriate quality systems incorporate cGMP standards and a quality-by-design approach—ensuring robust support from early development through clinical manufacturing.
Together, Agilent and BIOVECTRA offer a unified path from sequence to clinic—without the complexity of managing multiple CDMOs.
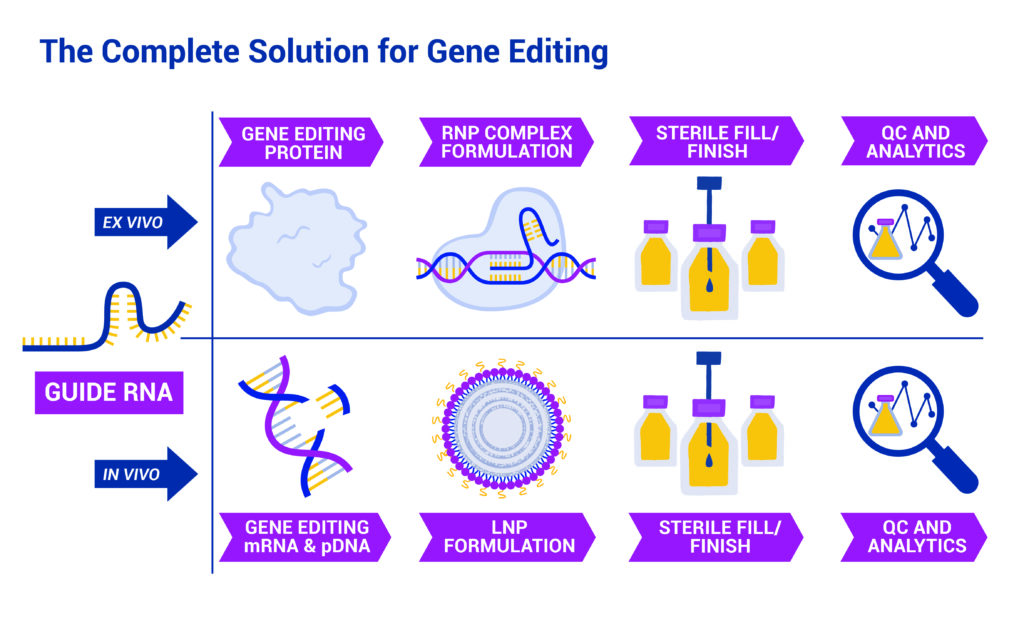
The complete solution for gene editing. Whether you’re producing an ex vivo or in vivo gene product, BIOVECTRA and Agilent can take it from the sequence to drug product.
We’re with you from sequence to clinic
Our team guides you every step of the way. The result? Expedited time to clinic, lower costs, and reduced risk.
Integration of all our gene-editing services:
- Minimizes contracts and quality agreements—to one agreement
- Improves slot availability and helps reduce the chance of penalties for delays
- Reduces shipment risks and costs
- Optimizes regulatory submissions
One unified team. One timeline. One purpose.
Let’s build your breakthrough, together
Contact us to design your custom manufacturing strategy.

Process Development
Demonstrated experience in process development and characterization, offering phase appropriate solutions and rapid optimization.

Analytical Development
Targeted analytical support from early development to validation of commercial processes and continuous process verification, all along the life cycle of your program.

Tech Transfer
Dedicated tech transfer teams ensuring a seamless transition from development to manufacturing - collaborating with our clients with a problem-solving mindset.

Clinical Development
Supplying our clients with pre-clinical and clinical material to enabling them to move quickly and cost-efficiently through the clinical phases.

Scale-Up
De-risking our client’s programs by effectively managing the transition from lab to pilot to manufacturing scale.

Commercial Scale
A proven history of commercial manufacturing partnerships growing alongside our clients. Effective collaboration increasing speed-to-market.