Dedicated to solving complex problems and managing increasingly demanding products—we’re committed to high potency API excellence
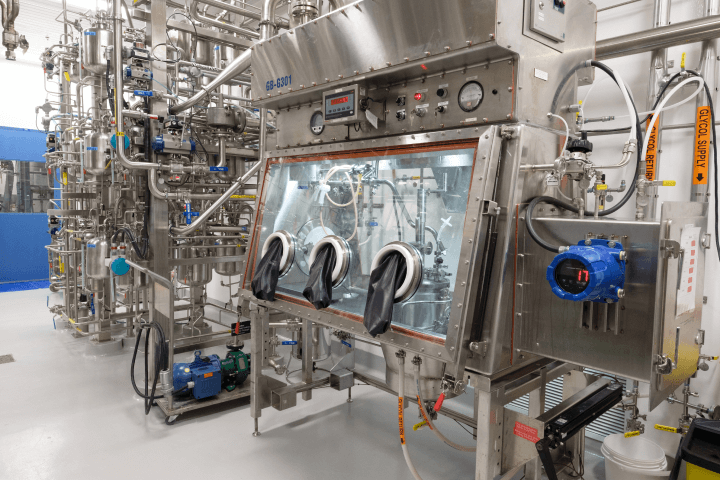
As the pharmaceutical industry improves highly potent therapeutic targeting using small and large molecule approaches, the demand for high-potency API (HPAPI) continues to increase. With extensive HPAPI process development and production experience for small and large molecule APIs, BIOVECTRA is here to advance your project. Complex products that require specific handling, conjugation, or other specialized processing are areas where BIOVECTRA shines.
We offer pilot-to-commercial-scale HPAPI capabilities, providing full support throughout the product life cycle.